Adrenal Problems in CFS
Addressing Hypothalamic-Pituitary-Adrenal Axis Dysfunction in Patients with Chronic Fatigue Syndrome (CFS) and Fibromyalgia (FM)
Points addressed:
- A review of the literature regarding evidence of significant hypothalamic-pituitary-adrenal axis (HPA) dysfunction in CFS and FM.
- Indications and efficacy of therapy with physiological doses of cortisol.
- Expected risks and benefits of such therapy.
Abstract:
There is clear evidence that adrenal axis dysfunction is present in patients with chronic fatigue syndrome (CFS) and fibromyalgia (FM) 1-21,23-28 and that therapy with low physiologic doses of cortisol have been shown to be safe, appropriate and effective.8,9,10,23,30 It should be considered the standard of care to help patients with CFS and FM who have baseline cortisol levels under 12 ug/ml.8,9,10,31,32,33
Evidence for significant HPA axis dysfunction with resultant adrenocortical dysfunction:
A study published in the Annals New York Academy of Sciences entitled Evidence for and Pathophysiologic Implications of Hypothalamic-Pituitary-Adrenal Axis Dysregulation in Fibromyalgia and Chronic Fatigue Syndrome discussed the evidence for HPA axis insufficiency in CFS and FM. They conclude, “Our group has established the impaired activation of the hypothalamic-pituitary-adrenal axis is an essential neuroendocrine feature of this condition.” 27
Cleare et al published a study in the American Journal of Psychiatry that obtained 24-hour urine collections from 121 consecutive patients with CFS. They found low 24 hour cortisol levels in all of the CFS patients. The authors conclude, “Urinary free cortisol was significantly lower in the subjects with chronic fatigue syndrome regardless of the presence or absence of current or past comorbid psychiatric illness…From whatever cause, low circulating cortisol is associated with fatigue; furthermore, raising cortisol levels can reduce fatigue in chronic fatigue syndrome. Thus, this study provides further evidence that adrenocortical dysfunction in chronic fatigue syndrome, whatever the etiology and whether primary or secondary, may be one piece of the multifactorial jigsaw underlying the production of symptoms in chronic fatigue syndrome.” 7 The authors agree that addressing this adrenocortical dysfunction with cortisol replacement is a fundamentally necessary part of the appropriate multi-system therapy of this condition.
Another study published in the Journal of Endocrinological Investigation performed a combination of stimulation tests on FM patients. They found over 95% of these patients had HPA axis dysfunction.3 They state, “The etiology and pathophysiology of this disease is not fully understood but the current data suggests that the PFS [Primary Fibromyalgia Syndrome] is not a primary disease of muscle. In contrast, an increasing amount of evidence suggests that the central stress axis, the HPA axis, seems to play an important role in the development of PFS…This study clearly shows that the HPA axis is underactivated in PFS...” 3
Trophy et al. administered interleukin-6 (IL-6), which is a potent stimulator of the HPA axis, and measured plasma ACTH and cortisol levels. They found a delayed ACTH response in these patients, a state that is consistent with a defect in the hypothalamic CRH neuronal function, as an etiology of symptoms in these patients.2
Cortisol levels normally increase with pain, but it has been shown that patients with CFS and FM either cannot appropriately increase cortisol production with pain or the inability to increase cortisol causes the increased pain. A study published in the November 2005 Journal Arthritis and Rheumatism demonstrated this strong relationship between cortisol levels and pain in individuals with CFS and FM and that low cortisol levels alone explained 38% of the variation in pain upon waking. The authors conclude, “The results of this study indicate that pain symptoms in women with FM are associated with cortisol concentrations during the early part of the day…These data support the hypothesis that HPA axis function is associated with symptoms in FM and accounts for the substantial percentage of pain symptom variance during the early part of the day.” 28
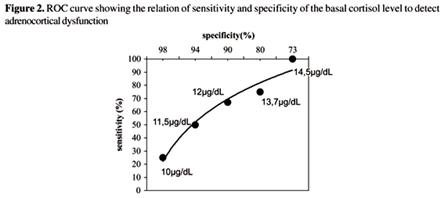
A study published in the Brazilian Journal of Infectious Disease (figure 2) demonstrates that in this type of patient population a baseline cortisol level of less than 12 has a specificity of greater than 90% for adrenocortical dysfunction and a level less than 10 ug/dl has a specificity of 98% for adrenocortical dysfunction.33 The most appropriate cutoff that optimizes specificity and sensitivity as found in this study as well as by others is 12 ug/dl.31,33 In addition, a normal ACTH does not rule-out secondary hypoadrenalism, but an abnormally low or low normal ACTH level can be considered confirmatory.
Dynamic testing:
Dynamic testing is certainly useful in straight forward primary and secondary adrenal insufficiency, but in patients with CFS, there is a complex interaction of hypothalamic and pituitary dysfunction. This results in a complex response that is initially elevated and then blunted, resulting in what is thought to be conflicting results, depending on which stimulation test was used, how an abnormal is defined and whether or not ACTH/cortisol ratios were used. 1,3,8,9,19,34-39 Consequently, the standard criteria does not apply to these patients significantly reducing the usefulness of dynamic testing with CFS and FM.1,9,19,27,34-39
It has been shown that a single ACTH stimulation test misses the majority of FM/CFS patients that have adrenocortical deficiency, but when a combination of stimulation tests are used, such as metyrapone test, or more sophisticated analysis is used, close to 100% of these individuals have documented adrenocortical dysfunction.1,3,8,9,19,27,34-39
Numerous studies demonstrate that this lack of sensitivity likely explains the seemingly contradictory findings between studies using stimulation tests.1,3,8,9,19,27,34-40 For instance, Scott et al in Clinical Endocrinology did 1ug ACTH stimulation tests on subjects with CFS and found a significant decrease in the delta cortisol value of patients with CFS vs. normals, but found that the reliance on this test and the arbitrary cutoffs that apply significantly impacts the sensitivity of stimulation tests in the patient population. They conclude, “In conclusion, the amount of cortisol released following stimulation with 1ug ACTH, is lower in CFS patients than in healthy volunteers…We propose, as has been suggested from previous studies, that the abnormality of HPA regulation is more likely to be central in origin. The demonstration of low basal ACTH in our CFS cohort [as in this case] would have supported this view…There is considerable debate surrounding the optimal dose of ACTH to use, with concern that the 250 mcg dose is “superphysiologic’ and may produce cortisol responses in patients suspected of having pituitary adrenal insufficiency that are falsely reassuring...Disparities between our healthy volunteer data and those of other groups using the 1 microgram ACTH test suggest that the test may not be as reliable as previously indicated…replacement therapy may more appropriately involve not only glucocorticoid, but mineralcorticoid supplements also.” 19
Another study that clearly demonstrates this confusion and that standard stimulation tests are not a reasonable method of evaluation of adrenal-cortical dysfunction is published in the Journal of Clinical Endocrinology and Metabolism entitled Evidence for Impaired Activation of the Hypothalamic Pituitary-Adrenal Axis in Patients with Chronic Fatigue Syndrome. The study found that compared to normal individuals, CFS patients were shown to have significantly reduced basal glucocorticoid levels (average 89 vs 148 nmol/l) and a low 24 hour urinary free cortisol excretion (122.7 nmol/L vs 203 nmol/L). The level of cortisol binding globulin CBG was also significantly higher in CFS patients making the free cortisol index lower in the patients (2.9 vs 8.9). There was a significant attenuated net integrated ACTH response to CRH but there was an initial increased initial sensitivity to ACTH with a reduced maximal response.1
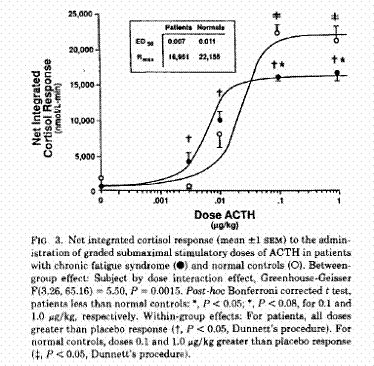
Although this cortisol response to ACTH is clearly abnormal for all of the patients with CFS, the dose response curve varies. There is an initial exaggerated response followed by an abnormally blunted response, which is not the case for patients with primary or secondary adrenocortical insufficiency without a dysfunctional hypothalamus. Consequently, standard dynamic testing is not medically useful in these patients and it is improper to use the defined normal cutoffs of response as is done with other conditions. This has been demonstrated in other studies as well.1,3,8,9,19,27,34-39
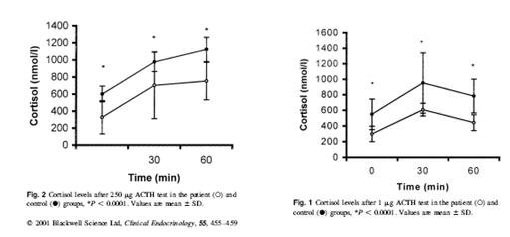
Kirnap et al in a study published in Clinical Endocrinology compared standard and low dose ACTH stimulation tests on patients with primary fibromyalgia syndrome (PFS). They found a significantly reduced peak cortisol response in the PFS verses controls. They also found that if the standard cutoff of 550 nmol/l was used with the standard ACTH stimulation, many of the patients would have misdiagnoses as normal.4
There are a number of theories that have been postulated to explain such a response. The author of this study postulates that this is due to a lack of CRH (corticotrophin releasing hormone) with a secondary hyperresponsivness from inadequate levels of ACTH. They alternatively state that this HPA axis defect could be secondary to a chronic viral infection.
Therapy:
Further supporting the use of low-dose cortisol in these patients is the fact that such therapy has been shown to improve the HPA axis response in these patients. This is counterintuitive to what physicians are taught and have found with higher pharmacological doses of glucocorticoids. In a study published in the 2001 Journal of Clinical Endocrinology & Metabolism entitled Hypothalamo-Pituitary-Adrenal Axis Dysfunction in Chronic Fatigue Syndrome, and the Effects of Low-Dose Hydrocortisone Therapy, the authors utilized ACTH and cortisol responses to CRH, insulin stress test, D-fenfluramine and 24 hour urinary free cortisol in 37 patients with CFS and prescribed these patients low-dose cortisol. They found that the therapy resulted in significant improvement and not only was there no adrenal suppression, but rather there was an improvement in the HPA axis as documented with CRH testing. They concluded, “In this group, there was a significant increase in the cortisol response to human CRH, which reversed the previously observed blunted responses seen in these patients. We conclude that the improvement in fatigue seen in some patients with chronic fatigue syndrome during hydrocortisone (same as cortisol) therapy is accompanied by a reversal of the blunted cortisol responses to human CRH.” 8
In a randomized, double-blind, placebo controlled, crossover, intent-to-treat trial published in The Lancet, patients with chronic fatigue syndrome were prescribed low dose hydrocortisone (5-10 mg/day) or placebo. The study found significant improvements in those prescribed low dose hydrocortisone vs. placebo and 28% improved to normal levels. The authors concluded, “This study shows that low-dose hydrocortisone results in significant reduction in self-rated fatigue and disability in patients with chronic fatigue syndrome…The degree of disability was reduced with hydrocortisone therapy, but not with placebo. Insulin stress tests showed that endogenous adrenal function was not suppressed by hydrocortisone.” 9 This demonstrates the effectiveness and appropriateness of this therapy.
Another randomized control trial published in JAMA also found significant improvement in fatigue scores with hydrocortisone replacement, but they used excessive dosing of 25-35 mg of cortisol. It is recommended that dosing be limited to 10-20 mg/day, as these doses have been shown to not be associated with any untoward effects and carries little to no risk of adrenal suppression.10,23,30,31 This considerable safety and negligible risk is also confirmed in endocrinology texts.54
Bashetti has published a number of studies on cortisol and CFS. He writes in the Journal of Endocrinology and Metabolism, “Hydrocortisone, the glucocorticoid that is routinely prescribed to correct the chronic cortisol deficiency of patients with Addison’s disease, has recently been confirmed to be significantly effective also in the therapy of chronic fatigue syndrome (CFS). This comes as no surprise if we consider that CFS and Addison’s disease share 26 features.” 41
A randomized, double blind placebo-controlled, intent-to-treat study by Teitelbaum published in the Journal of Chronic Fatigue Syndrome documented the effectiveness of an integrative therapy approach to CFS and FM that includes low-dose cortisol (7.5-20 mg/day). The authors conclude,” Significantly greater benefits were seen in the active group than in the placebo group for all primary outcomes. Using an integrated therapy approach, effective therapy is now available for FMS/CFS.” 31
A subsequent editorial in the peer reviewed, Journal of the American Academy of Pain Management reviewed this study and agreed that an integrative approach that includes low-dose cortisol is the standard of care for these conditions. The author states, “The study by Dr. Teitelbaum et al. and years of clinical experience makes this approach an excellent and powerfully effective part of the standard of practice for therapy of people who suffer from FMS and MPS [myofacial pain syndrome]— both of which are common and devastating syndrome.” 48 The consensus opinion among those who are experts in addressing CFS and FM is that a therapy approach that includes low-dose cortisol is the standard of care.
A subsequent commentary by Teitelbaum published in JAMA states, “Our previously published pilot study and the work of Jefferies suggests that using low-dose hydrocortisone in CFS as dosages of 7.5 mg to 20 mg/day is safe and effective. These low dosages have not caused adrenal suppression…We recently completed a randomized, double-blind study that tested the effectiveness of prescribing therapy for hypothalamic dysfunction in an integrated manner for patients with fibromyalgia and CFS. This included addressing suspected hormonal deficiencies (including low hydrocortisone) and the sleep disorder simultaneously. Using this protocol in 72 patients resulted in a significant improvement in active vs. placebo group.” 42 Cortisol replacement appears to be an essential part of a comprehensive therapy approach that can be used successfully in addressing CFS and FM.31,42
A study published in JAMA found that nearly half of the patients prescribed mineralcorticoid reported complete or nearly complete resolution of CFS symptoms.43
The safety of low dose glucocorticoids was addressed in a 48 page review article published in last month’s Annals of Rheumatic Diseases entitled Low-dose glucocorticoid therapy in rheumatoid arthritis- A review on safety: published evidence and prospective trial data. This extensive review assessed the incidence and severity of adverse effects of long-term low-dose glucocorticoid therapy in rheumatoid arthritis. This review considered low dose as any dose below or equivalent to 40 mg hydrocortisone (this patient received a fraction of this dose 10mg/day). They concluded, “Adverse-effects of glucocorticoids are abundantly referred to in literature. However, in the available literature on low-dose glucocorticoid therapy very little of the commonly held beliefs about their incidence, prevalence and impact of GC [glucocorticoid] proved to be supported by clear scientific evidence. Additional data from the randomized controlled clinical trials reviewed showed that the incidence, severity and impact of adverse effects of low dose glucocorticoid therapy in rheumatoid arthritis trials are modest, and often not statistically different to those of placebo.” 10
Low-dose cortisol has been shown to improve immunity, as opposed to the well known immunosuppressive effect of pharmacological doses of glucocorticoids, 23,30,45,46 and has been shown to improve recovery from chronic infections such as EBV.47,22,29
In summary, it is becoming clear that the majority of patients with CFS and FM suffer from clinically significant adrenocortical dysfunction and that physiologic replacement of cortisol is an appropriate intervention in these patients. Cortisol doses of 15-20 mg/day have been shown to be safe, with little associated risk including adrenal suppression, and have the potential for significant clinical benefit. The current evidence supports the use of physiologic doses of cortisol in addressing CFS and FM, and a therapeutic trial of cortisol should be considered in these patients, especially those with basal cortisol levels less than 12 ug/dl.
References
1. Demitrack MA, Dale JK, Straus SE, Laue L, Listwak SJ, Kruesi MJP, et al. Evidence For Impaired Activation of the Hypothalamic-Pituitary-Adrenal Axis in Patients With Chronic Fatigue Syndrome. J Clin Endocrinol Metab 1991;73:1224-1234.
2. Torpy DJ et al. Responses of the sympathetic nervous system and the hypothalamic-pituitary-adrenal axis to interleukin-6 in fibromyalgia. Arthritis and Rheumatism. 2000; 43: 872-880.
3. Calis M, Gokce C. Investigation of the hypothalamo-pituitary-adreanl axis (HPA) by 1ug ACTH test and metyrapone test in patients with primary fibromyalgia syndrome. J Endocrinol Invest 2004 27:42-46
4. Kirnap M, Colak REser C, Ozsoy OTutus A, Kelestimur F. A comparison between low-dose (1 microg), standard-dose (250 microg) ACTH stimulation tests and insulin tolerance test in the evaluation of hypothalamo-pituitary-adrenal axis in primary fibromyalgia syndrome. Clin Endocrinol (Oxf). 2001 Oct;55(4):455-9
5. Griep EN, Boersma JW, de Kloet ER. Altered Reactivity of the Hypothalamic-Pituitary-Adrenal Axis in the Primary Fibromyalgia Syndrome. J Rheumatol 1993;20:469-74
6. Jens Gaab, PhD, Dominik Hüster, MSc, Renate Peisen, MSc, Veronika Engert, BSc, Vera Sheitz, BSc, Tanja Schad, BSc, Thomas H. Schürmeyer, PhD, MD and Ulrike Ehlert, PhD. Hypothalamic-Pituitary-Adrenal Axis Reactivity in Chronic Fatigue Syndrome and Shealth Under Psychological, Physiological, and Pharmacological Stimulation. Psychosomatic Medicine 64:951-962 (2002)
7. Cleare AJ, Blair D, Chambers S, Wessely S, Urinary Free Cortisol in Chronic Fatigue Syndrome Am J Psychiatry 158:641-643, April 2001
8. Cleare A et al. Hypothalamo-Pituitary-Adrenal Axis Dysfunction in Chronic Fatigue Syndrome, and the Effects of Low-Dose Hydrocortisone Therapy. The Journal of Clinical Endocrinology & Metabolism 2001. 86(8):3545–3554.
9. Cleare AJ et al. Low-dose hydrocortisone in chronic fatigue syndrome: a randomized crossover trial. Lancet 1999 Feb 6;353(9151):455
10. Gaab J, Huster D, Peisen R, Engert V, Schad T, Schurmeyer TH, Ehlert U. Low-dose dexamethasone suppression test in chronic fatigue syndrome and health. Psychosom Med. 2002 Mar-Apr;64(2):311-8
11. Altemus M, Dale JK, Michelson D, Demitrack MA, Gold PW, Straus SE. Abnormalities in response to vasopressin infusion in chronic fatigue syndrome. Psychoneuroendocrinology 2001 Feb 1;26(2):175-188
12. Scott LV, Svec F, Dinan T. A preliminary study of dehydroepiandrosterone response to low-dose ACTH in chronic fatigue syndrome and in healthy subjects. Psychiatry Res 2000 Dec 4;97(1):21-28
13. Scott LV, Teh J, Reznek R, Martin A, Sohaib A, Dinan TG. Small adrenal glands in chronic fatigue syndrome: a preliminary computer tomography study. Psychoneuroendocrinology 1999 Oct;24(7):759-68
14. Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000 Jan;25(1)
15. Scott LV, Medbak S, Dinan TG. Desmopressin augments pituitary-adrenal responsivity to corticotropin-releasing hormone in subjects with chronic fatigue syndrome and in healthy volunteers. Biol Psychiatry 1999 Jun 1;45(11):1447-54
16. De Becker P, De Meirleir K, Joos E, Campine I, Van Steenberge E, Smitz J, Velkeniers B Dehydroepiandrosterone (DHEA) response to i.v. ACTH in patients with chronic fatigue syndrome. Horm Metab Res 1999 Jan;31(1):18-21
17. Z Crofford L. The hypothalamic-pituitary-adrenal stress axis in fibromyalgia and chronic fatigue syndrome. J Rheumatol 1998;57 Suppl 2:67-71
18. Kuratsune H, Yamaguti K, Sawada M, Kodate S, Machii T, Kanakura Y, Kitani T. Dehydroepiandrosterone sulfate deficiency in chronic fatigue syndrome. Int J Mol Med 1998 Jan;1(1):143-6
19. Scott LV, Medbak S, Dinan TG The low dose ACTH test in chronic fatigue syndrome and in health. Clin Endocrinol (Oxf) 1998 Jun;48(6):733-7
20. Scott LV, Medbak S, Dinan TG. Blunted adrenocorticotropin and cortisol responses to corticotropin-releasing hormone stimulation in chronic fatigue syndrome. Acta Psychiatr Scand 1998 Jun;97(6):450-457
21. Strickland P, Morriss R, Wearden A, Deakin B A comparison of salivary cortisol in chronic fatigue syndrome, community depression and healthy controls. J Affect Disord 1998 Jan;47(1-3):191-194
22. Bender CE. The value of corticosteroids in addressing infectious mononucleosis. JAMA 199;529, 1967
23. Jefferies W. Mild adrenocortical deficiency, chronic allergies, autoimmune disorders and the chronic fatigue syndrome: a continuation of the cortisone story, Med Hypotheses, 1994, Issue: 3, Volume: 42, Page: 183-9, ISSN: 0306-9877
24. Cleare AJ; Bearn J; Allain T; McGregor A; Wessely S; Murray RM; O'Keane V. Contrasting neuroendocrine responses in depression and chronic fatigue syndrome, J Affect Disord, 1995 Aug 18, Issue: 4 Volume: 34 Page: 283-9
25. Moutschen M; Triffaux JM; Demonty J; Legros JJ; Lefèbvre PJ; . Pathogenic tracks in fatigue syndromes Acta Clin Belg, 1994, Issue: 6 Volume: 49 Pagination: 274-89 ISSN: 0001-5512
26. Carruthers et al. Myalgic Encepalomyelitis/Chronic Fatigue Syndrome: Clinical Working Case Definition, Diagnostic and Therapy Protocols. Journal of Chronic Fatigue Syndrome.Vol 11(1) 2003
27. Demitrack MA, Crofford LJ. Evidence for and pathophysiologic implications of hypothalamic-pituitary-adrenal axis dysregulation in fibromyalgia and chronic fatigue syndrome. Ann N Y Acad Sci 1998 May 1;840:684-697
28. Samuel A. McLean,1 David A. Williams,1 Richard E. Harris,1 Willem J. Kop,2 Kimberly H. Groner,1 Kirsten Ambrose,1 Angela K. Lyden,1 Richard H. Gracely,1Leslie J. Crofford,3 Michael E. Geisser,1 Ananda Sen,1 Pinaki Biswas,1 and Daniel J. Clauw1. Momentary Relationship Between Cortisol Secretion and Symptoms in Patients With Fibromyalgia. Arthritis & Rheumatisim Vol. 52, No. 11, November 2005, pp 3660–3669
29. Manji RJ et al. Depression of cell-mediated immunity durng acute infectious mononucleosis. N Engl J Med 291:1149, 1974
30. Jefferies W. Cortisol and Immunity. Medical Hypotheses 1991;34:198-208
31. Teitelbaum J, Bird B, Greenfield R, Weiss A, Muenz L, Gould L. Effective Therapy of Chronic Fatigue Syndrome (CFIDS) & Fibromyalgia (FMS) - A Randomized, Double-Blind, Placebo-Controlled, Intent-to-Treat Study. Journal of Chronic Fatigue Syndrome Volume 8, Issue 2 – 2001
32. Takeshita S et al. Intravenous immunoglobulin preparations promote apoptosis in lipopolysaccharide-stimulated neutrophils via an oxygen-dependent pathway in vitro. APMIS 2005:113:269-77.
33. Wolff FH, Nhuch C, Cadore LP, Glitx CL, Lhullier F, Furlanetto TW. Low-dose adrenocorticotropin test in patients with the Acquired Immunodeficiency Syndrome. Braz. J. Infect. Dis. Apr. 2001, vol.5, no.2
34. Tordjman K., Jaffe A., Grazas N. et al. The role of the low dose (1 mg) adrenocorticotropin test in the evaluation of patients with pituitary diseases. J Clin Endocrinol Metab 1995; 80:1301 5.
35. Dickstein G., Schechner C., Nicholson W.E., et al. Adrenocorticotropin stimulation test: effects of basal cortisol level, time of the day, and suggested new sensitive low dose test. J Clin Endocrinol Metab 1991;72:773-8
36. Crowley S., Hindmarsh P.C., Honour J.W., Brook C.G.D. Reproducibility of the cortisol response to stimulation with a low dose of ACTH (1-24): the effect of basal cortisol levels and comparison of low dose with high dose secretory dynamics. J Endocrinol 1993;136:167-72
37. Baraia-Etxaburu Artetxe J., Astigarraga Aguirre B., Elorza Olabegova R., et al. [Primary adrenal failure and AIDS: report of 11 cases and review of the literature]. Rev Clin Esp 1998;198:74-9.
38. Zarkovic M., Ciric J., Stojanovic M., et al. Optimizing the diagnostic criteria for standard (250-mg) and low dose (1-mg) adrenocorticotropin tests in the assessment of adrenal function. J Clin Endocrinol Metab 1999;84:3170-3.
39. Abdu TA, Elhadd T.A., Neary R., Clayton R.N. Comparison of the low dose short synacthen test (1 mg), the conventional dose short synacthen test (250 mg), and the insulin tolerance test for assessment of the hypothalamo-pituitary-adrenal axis in patients with pituitary disease. J Clin Endocrinol Metab 1999;84:838-43.
40. Courtney CH et al. Authors’ Response: HPOA Axis Testing after Pituitary Surgery. Journal of clinical Endocrinology and Metabolism 2005;90:6744
41. Riccardo Baschetti, M.D. Investigations of Hydrocortisone and Fludrocortisone in addressing Chronic Fatigue Syndrome The Journal of Clinical Endocrinology & Metabolism Vol. 84, No. 6 2263-2264
42. Teitelbaum et al. To the Editor re: McKenzie et al. Low-Dose Hydrocortisone for Chronic Fatigue Syndrome. 281 No. 20, May 26, JAMA,1999 Vol. 281 No. 20, May 26, 1999. JAMA May 26, 1999 (281)20:1888
43. Bou-Holaigah et al. the relationship between neurally mediated hypotension and the chronic fatigue syndrome. JAMA 1995;274:961-7
44. Bashetti R. hydrocortisone and chronic Fatigue Syndrome. Lancet 1999;353:1618
45. Grayson J et al. Immunoglobulin production induced in vitro by glucocorticoid hormones:T-cell dependent stimulation of immunoglobulin production without B cell proliferation in cultures of human peripheral blood lympocytes. J clin Invest 68:1539,1981
46. Jeroen TJ et al. Altered Glucocorticoid Regulation of the Immune Response in the Chronic Fatigue Syndrome. Annals of the New York Academy of Sciences 917:868-875 (2000)
47. Chappel MR. Infectious mononucleosis. Southwest Med 43:253,1962
48. Blatman H, Effective Therapy of Fibromyalgia and Mypfacial Pain Syndrome: A Clinician’s Perspective. Journal of the American Academy of Pain Management. April 2002.

Jacob Teitelbaum, M.D. is one of the world's leading integrative medical authorities on fibromyalgia and chronic fatigue. He is the lead author of eight research studies on their effective treatments, and has published numerous health & wellness books, including the bestseller on fibromyalgia From Fatigued to Fantastic! and The Fatigue and Fibromyalgia Solution. Dr. Teitelbaum is one of the most frequently quoted fibromyalgia experts in the world and appears often as a guest on news and talk shows nationwide including Good Morning America, The Dr. Oz Show, Oprah & Friends, CNN, and Fox News Health.